The message from the parent was clear and with lots of documentation
My 5 y.o. daughter is having major gut issues for which I am seeking your help. She is having these issues since her birth and we just stand helpless while she cries most of the night. I have made an excel sheet explaining all her issues for your reference. I am also attaching her Biomesight.csv file, HTMA Test, GI Map test and her other medical test and stool test results for your kind reference.
| Gut Issues | Sensory Issues | General Behaviour | Socialization | Speech | History, Curent Supplements & Diet |
| Excessive Gas mostly in evening and night. | Hitting Hard Objects/Plastic in the front teeth | No aggression , no self injurious behaviours | Doesn’t have age appropraite play behaviour | No true words. Only few nonsense syllables | Birth weight-3.45 kg. C-Section delivery. |
| Bloating | Crashing & Jumping on couch & bed. | Sweet frendly kid. Attention seeker. | More afectionate to adults than kids. | Started comprehending two word commands with gestures | She had several doses of Antibiotics due to flu, fever on more than 5 occasions. She had a teeth infection for which she was given antibiotics. She also had some gut bug for which she vomited more than 12 times in a single night, in the past (at the age of 20 months) |
| Fatulence/Burps | Looking from the corner of the eye. | Scratching others | Near Normal Eye Contact and Sitting behaviour. | Child expresses her needs through hand leading and dragging parents towards the desired object. | Child gained a lot of weight since childhood. She was 22kg at the age of 2.5 years. Now she is 40kg. Her height is 120 cm |
| Undigested food in stool mostly vegetables | Rocking back & forth while standing. | Biting other | Poor Focus & Attention | Celiac test is negative. Low in Vitamin D & Iron. Ultrasound test of abdomen is normal | |
| Constipation. Bristol Stool Chart No.-3/4 | Making sudden loud noises | Pinching & Hitting others | Shows no interest/high resistance to any learning activity. Escape behaviour | Supplement– SunFiber, Liver Sauce, Chamomile Tea, Sodium BiCarbonate to relieve gas issues. | |
| There is immediate relief as soon as she passes the gas through fart or burps. | Unusual laughter/ Teeth Grinding (Occassionally) | Restlessness, Crying and Yelling | Diet -GFCFSF diet. Vegetables- Okra, potato, carrots, beans, tomato,snow peas. Other food- boiled chicken, low GI- rice, millet, gluten free flour, gluten/diary free cookies. Fruits- Banana, kiwi, apples. | ||
| Stifening of whole body and hitting with her finger tips | All the above problematic behaviour is noticed when the kid has gut issues | ||||
| Hitting her face on gym ball/mattress | |||||
| Hand Twirling infront of her face. |
Initial Review
My expertise is statistics associated with the microbiome, not autism (although I am a high functioning ASD person).
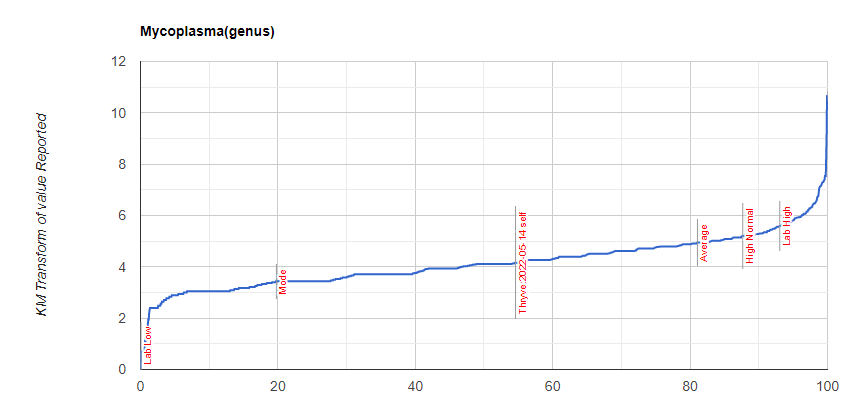
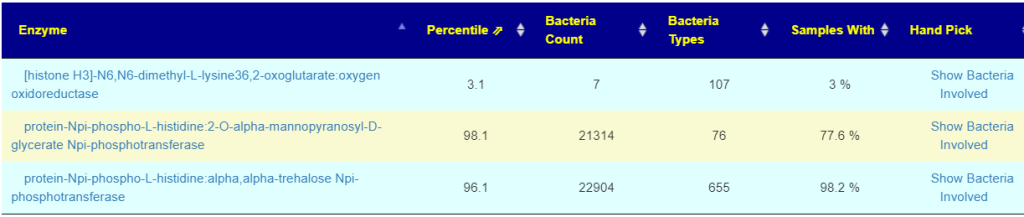
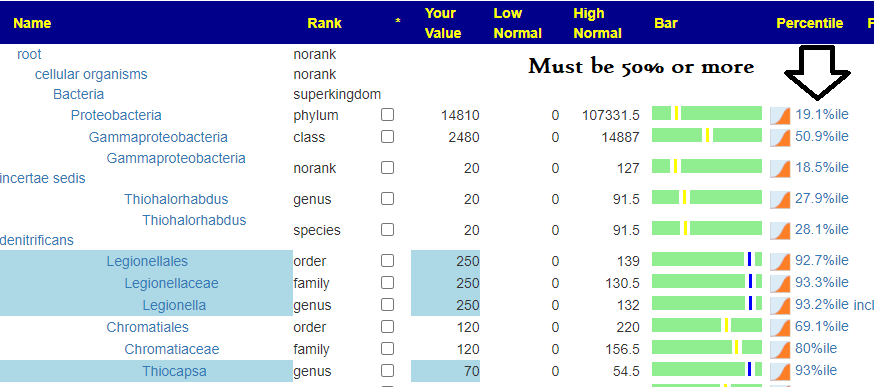
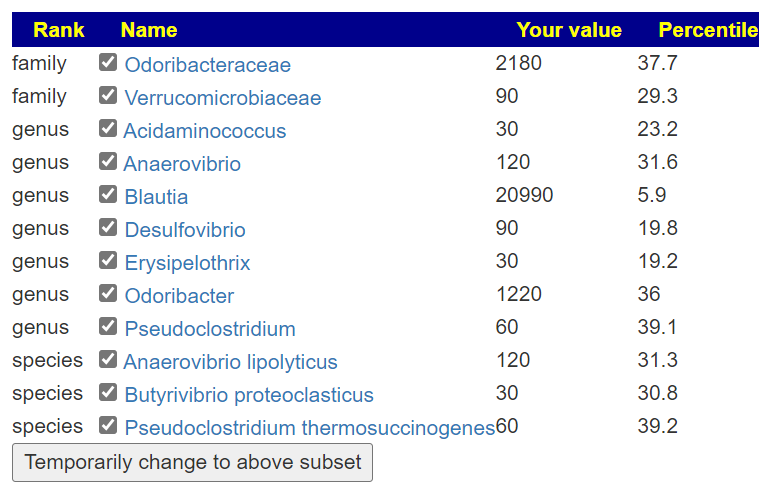
First looking at distributions we see a predominance of rare bacteria. Usually that hints at herbs to reduce their numbers. Dr. Jason Hawrelak Recommendations placed her at the 95.6%ile, so a main stream solution is unlikely.
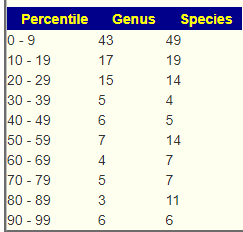
Looking at the unhealthy bacteria, several stands out as items of concern:
- Escherichia coli – which are likely the unhealthy ones. This immediately causes me to suggest either Mutaflor (available where she lives) or Symbioflor-2, the good E.Coli probiotics. Given her age, I would start with Symbioflor-2 because it is given in drops and thus we can slowly ramp up (to avoid severe adverse effects). The other option would be to repackage one capsules of Mutaflor in eight capsules and start with lower dosages. Given the symptoms above — this would be my first course of action if it was my child.
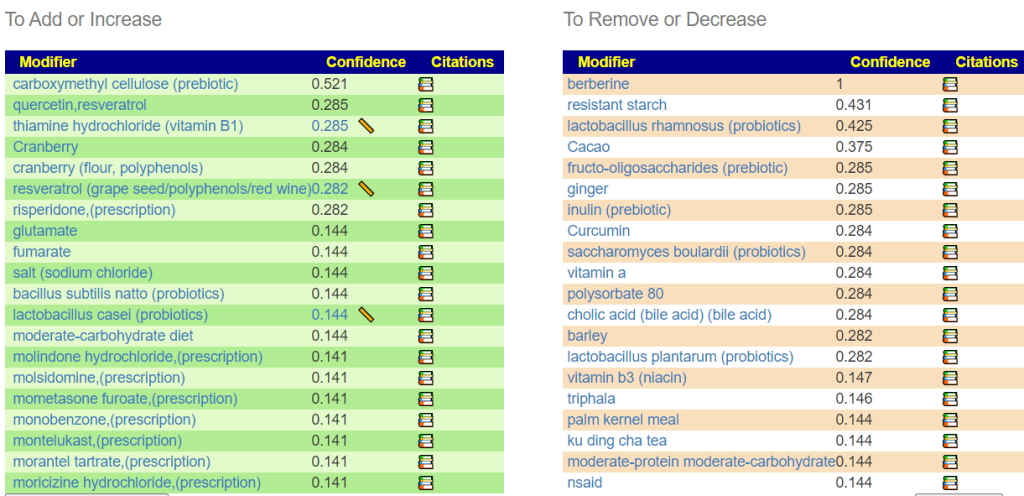
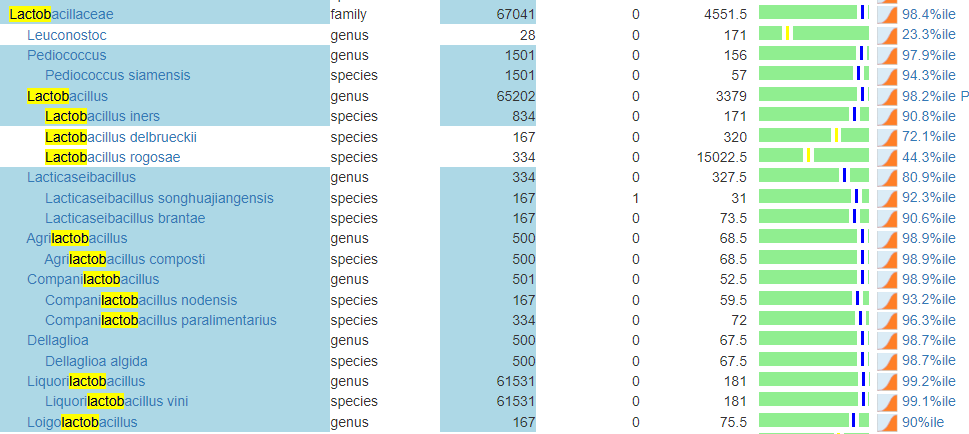
- Bacteroides fragilis is high. In terms of non-prescription, pomegranate, lactobacillus reuteri (probiotics), N-Acetyl Cysteine (NAC), Cacao and Mangosteen are the most documented. REMEMBER: There is no literature on relative effects, the Confidence on suggestions is based on the number of studies showing desired effects on the number of bacteria we selected to alter.
- Serratia is also high. Usually associated with urinary tract infections. For this, we have less literature. I would suggest neem tea and perhaps lactobacillus casei (probiotics)
At this point we have a little dilemma — Escherichia coli and Lactobacillus are hostile to each other, so it’s an either/or. I would usually resolve it by 2 weeks on one and then 2 weeks on the other, recording any changes seen.
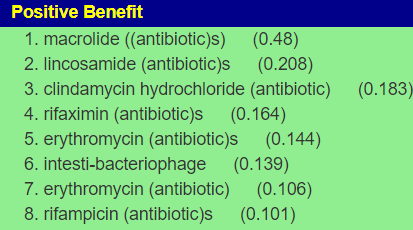
Given the history of needing antibiotics in the past, I looked at the computed suggestions. If the needs arise again, see if your MD is willing to use any of the following:
- macrolide ((antibiotic)s) (0.444)
- imipenem (antibiotic)s (0.382)
- fluoroquinolone (antibiotic)s (0.337)
- ciprofloxacin (antibiotic)s (0.305)
- clindamycin (antibiotic)s (0.294)
- erythromycin (antibiotic) (0.274)
Please make sure that you check risk factors for the above, especially given her age. For example, fluoroquinolone has many! The above were calculated solely on the microbiome impact, not risk factors.
Other Medical Reports
After the first impressions above, I went to look at the other test results
- Hair shows slightly out of range for Arsenic (common for ASD)
- Celiac tests: negative
- Cortisol: in range
- Lipid Profile: normal
- Serum Free T3+Free T4+TSH: normal
- Ultrasound: normal
- Vitamin D + Iron: normal
- Gastrointestinal Pathogen PCR (Stool): Negative
- Liver Function: normal except for
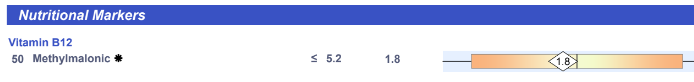
- Alkaline Phosphatase: high (2x upper limit)
- Sodium and Potassium – slightly high (similar on hair above)
- GI Map
- Bacteroides fragilis: High, as above
- Enterococcus: High (versus 18%ile, low on Biomesight results)
- Escherichia species: At top of reference range (97%ile on Biomesight results)
- Akkermansia Munciniphila: none detected (none detected on Biomesight results)
- Zonulin [literature] was very high indicating leaky gut.
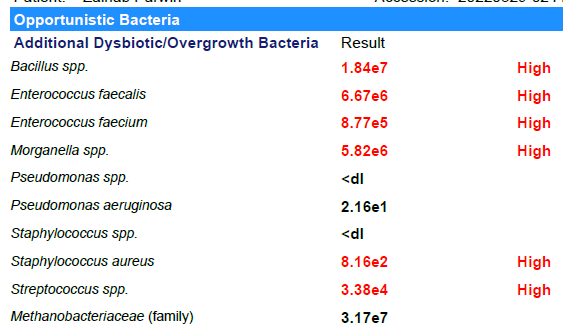
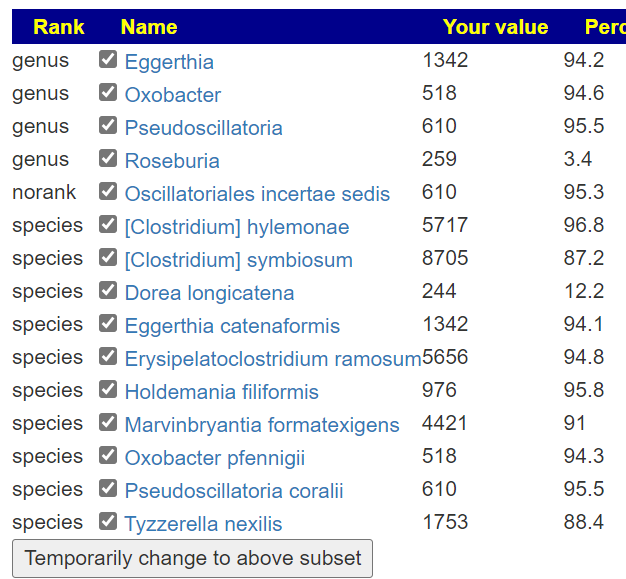
Looking at Biomesight results
- Bacillus was not high
- None of the Enterococcus were reported and the family was a little low
- Morganella was not reported
- S. Aureus was not reported
- Streptococcus was low
I will make the assumption that at least one round of antibiotics were done between the GI Map (Mar 29,2022) results and the Biomesight results — although I have read studies questioning the reliability of some GI Map results.
Probable Symptoms
This was recently revised, and seems to match — especially the time since offset.
| Symptom | Matches |
|---|---|
| less than 04 years since onset | 77 |
| Frequently loose train of thought | 75 |
| Lyme | 74 |
| Less Avoidance of Eye Contact or Poor Eye Contact | 73 |
Where Do we go from here?
I picked the following to build the consensus report shown below:
- Special Studies: Autism (75)
- Special Studies: Inflammatory bowel disease ( 86 candidate bacteria) – given all of the bowel issues
- JasonH (15 Criteria) – just 7
- Standard Lab Ranges (+/- 2 Std Dev) – just 7
- Box Plot Whisker – 45
- Kaltoft-Moltrup Normal Ranges – 109
- Percentile in top or bottom 10% – 134
It was interesting to note that the methods that selected the most bacteria, were the special studies, KM and the top/bottom 10%.
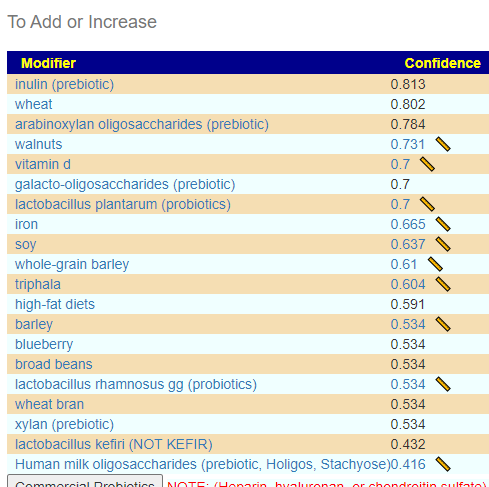
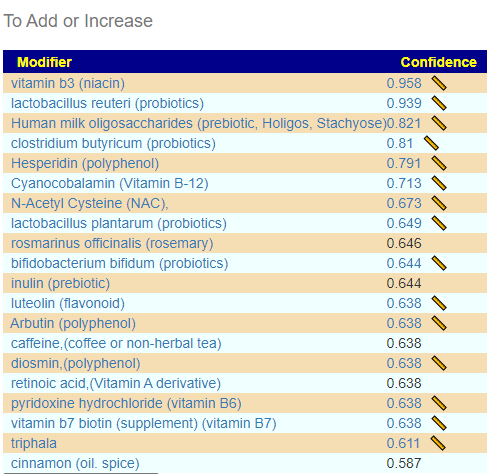
Consensus Suggestions
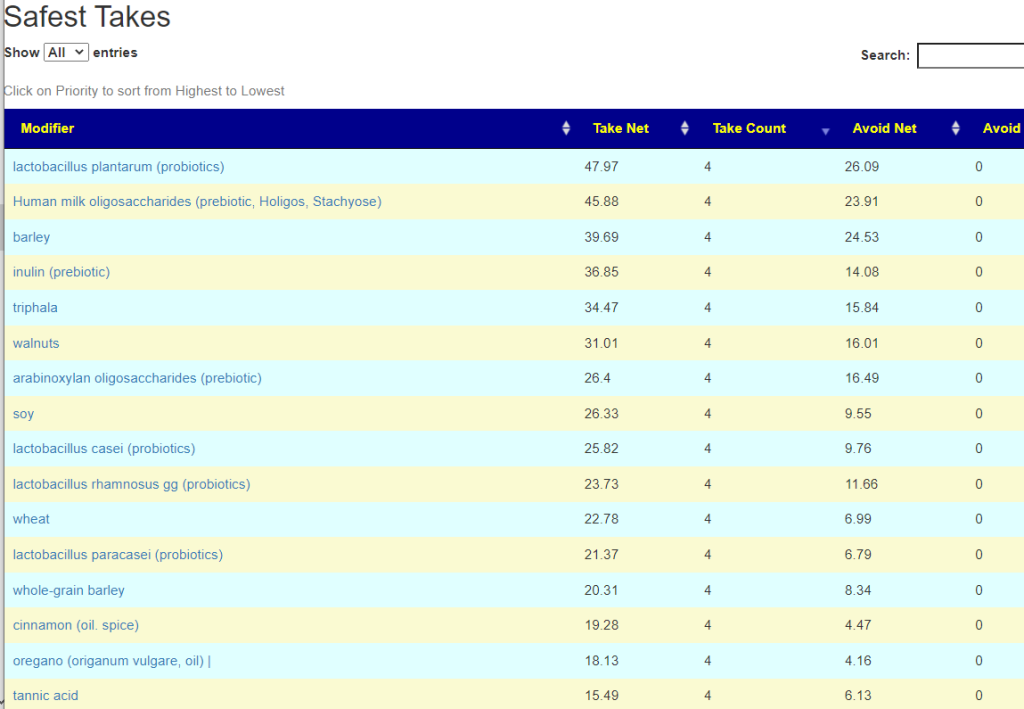
As always, the full consensus report is attached.
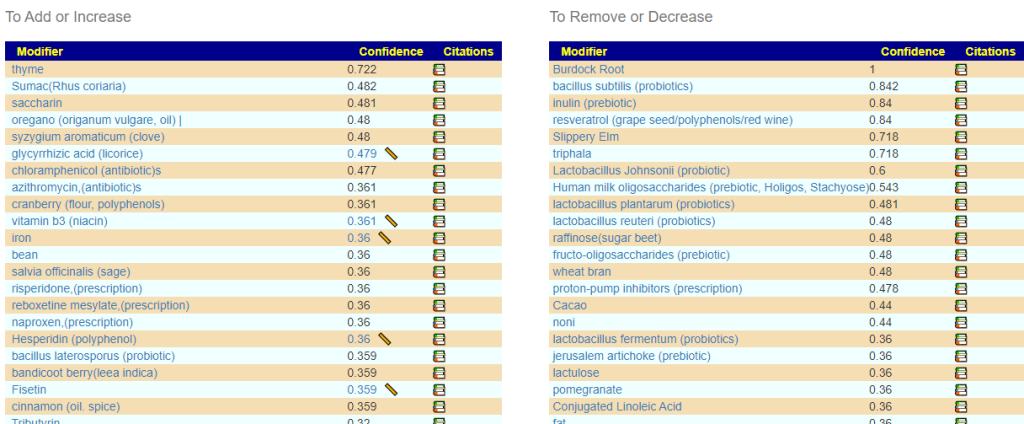
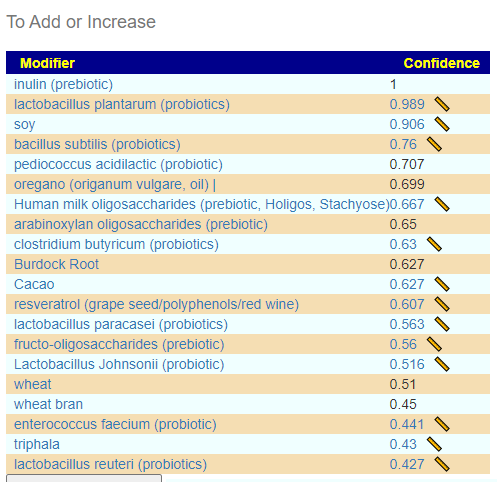
In reviewing the list, the ones that I would be most inclined to use are:
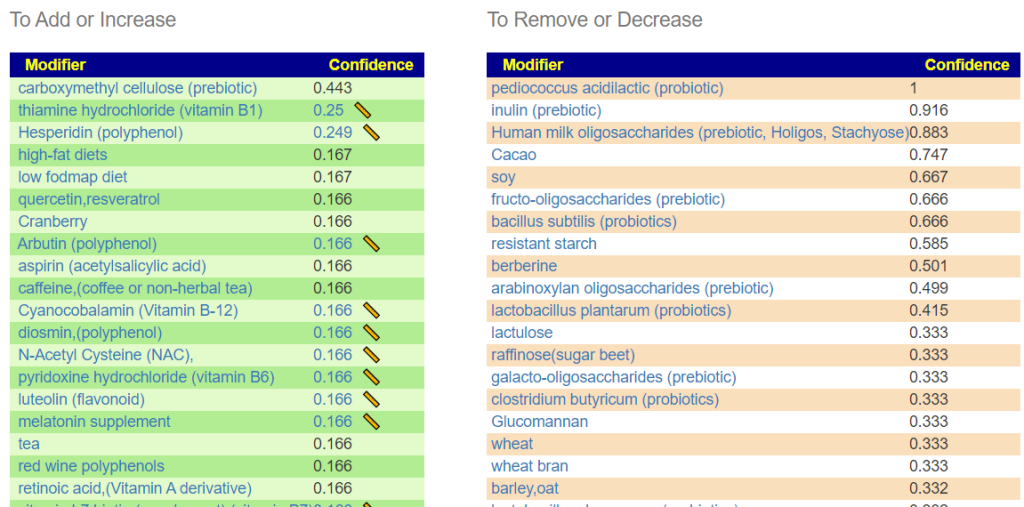
- Triphala
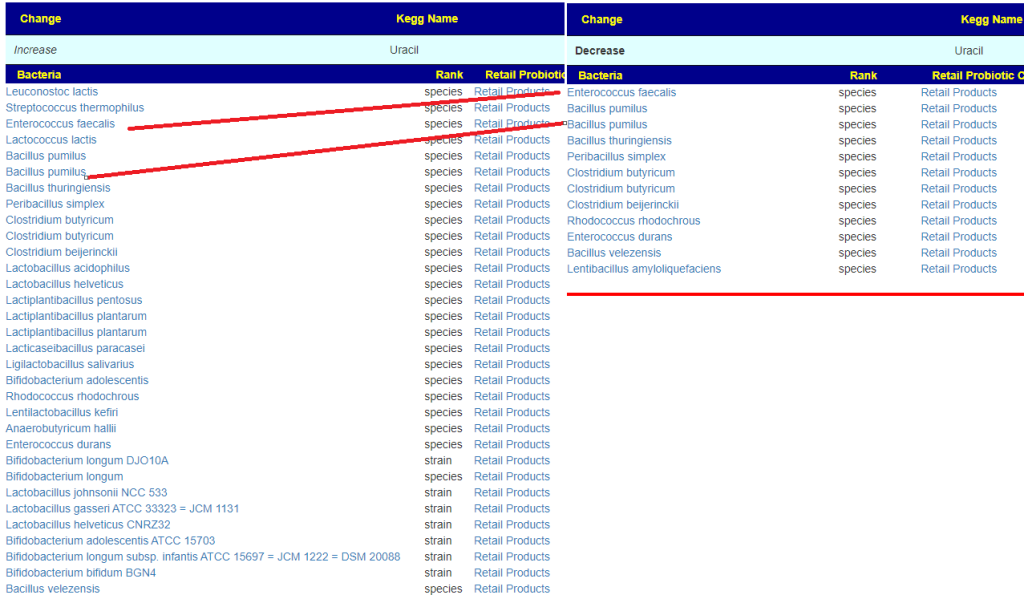
- Bofutsushosan (which will encourage Akkermansia!)
- Omega-3 fatty acids
- Lactobacillus rhamnosus (probiotics),
- berberine
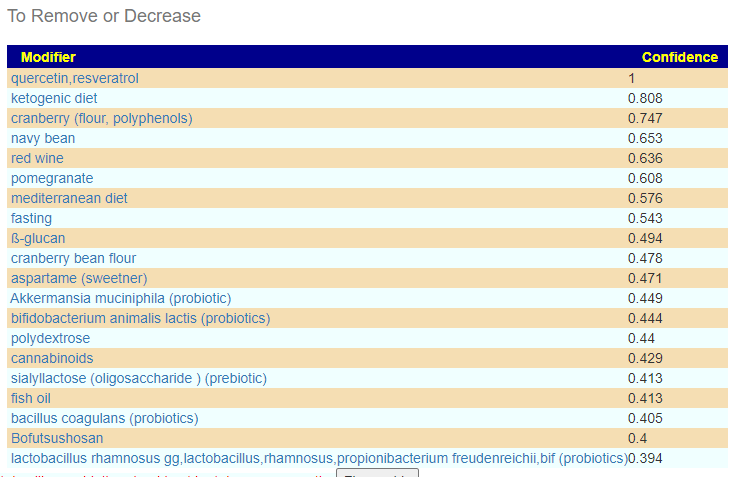
I should note that symbioflor 2 e.coli probiotics is on the avoid list — this often happens when there is a need to increase lactobacillus which was sitting at 6%ile. When lactobacillus is low, I tend to ignore the avoid on E.Coli probiotics and point out the mutual hostility they have – so do one and then the other, noticing any changes (the one that gives the best positive change, do more of — but keep rotating). I usually suggest starting with the E.Coli probiotics because they are known to persist. This is rarely the case for Lactobacillus probiotics.



Recent Comments